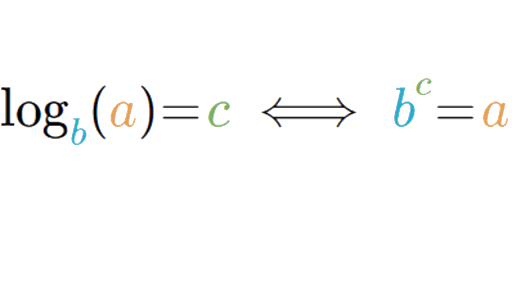 Learn about the properties of logarithms that help us rewrite logarithmic expressions, and about the change of base rule that allows us to evaluate any logarithm we want using the calculator.
Learn about the properties of logarithms that help us rewrite logarithmic expressions, and about the change of base rule that allows us to evaluate any logarithm we want using the calculator.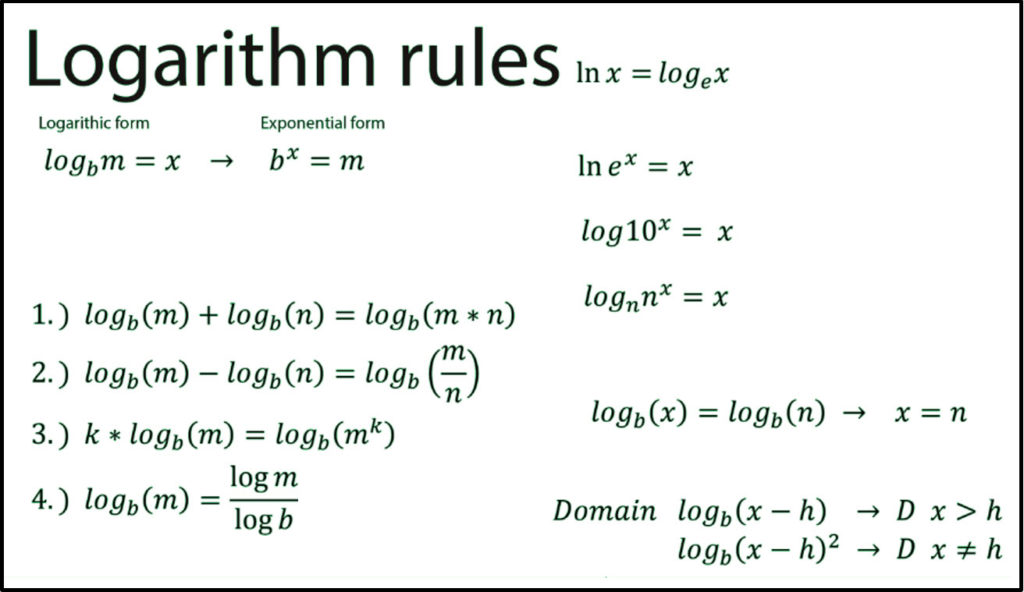 The pH scale is logarithmic, which means that a change of 1 pH unit represents a tenfold change in acidity or basicity. For example, a solution with a pH of 4 is ten times more acidic than a solution with a pH of 5.
The pH scale is logarithmic, which means that a change of 1 pH unit represents a tenfold change in acidity or basicity. For example, a solution with a pH of 4 is ten times more acidic than a solution with a pH of 5.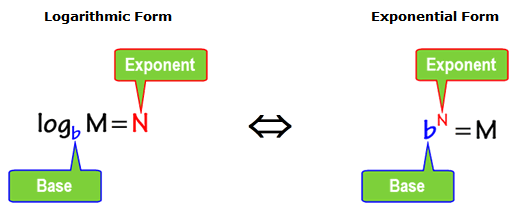 Understanding this basic concept can help us solve some algebra problems that require switching from one form to another. Let’s examine further how the variables M, N, and b are rearranged when the logarithmic form is expressed as exponential form and vice versa.
Understanding this basic concept can help us solve some algebra problems that require switching from one form to another. Let’s examine further how the variables M, N, and b are rearranged when the logarithmic form is expressed as exponential form and vice versa.